Abstract
The suggestion1,2 that the colloidal-dispersion-gel (CDG) process is superior to normal polymer flooding is misleading and generally incorrect. Colloidal dispersion gels, in their present state of technological development, should not be advocated as an improvement to, or substitute for, polymer flooding.
The Controversy
- February 2006 JPT
- Controvery Background
- Polymers and Gelants Can Flow Through Rock. Gels Do Not.
- Chang’s Claim Violates Darcy’s Law.
- Polymers and Gels Plug Low-k Rock More than High-k Rock
Laboratory Results
- Many Previous Lab Tests Show No Effect of Aluminum on Polymer.
- Other Lab Tests Show Permeability Reductions, But Gelation Times Are Short.
- Parallel Corefloods Are a Poor Indicator of Diversion.
- The Vendor's Lab Tests Show No Surprises.
- Requirements for a Viable Colloidal Dispersion Gel.
Examination of Field Data
- The Field and Lab Data Are Right. Only Interpretations Are Wrong.
- Field Results Can Be Explained Using More Plausible Concepts.
- Daqing Field Results Are Consistent with No Benefit from Aluminum Citrate.
- More Convincing Field Data Needed.
Searching for a New Mechanism of Action for CDG Gels
- Does Shearing Of Colloidal Dispersion Gels Allow For Effective, Deep Penetration?
- Can CDG Gels Flow in Porous Media with Permeabilities Less than 8 Darcys?
- Can CDG Gels Be More Effective than Normal Gels in Tight Fractures?
The Controversy
February 2006 JPT
In the February 2006 issue of the Journal of Petroleum Technology (JPT) H.L. Chang et al.1 summarized some of the pilot and commercial-scale field activities on polymer flooding and ASP flooding that were performed in China. Unquestionably, polymer flooding and ASP flooding can be effective oil-recovery processes and have great potential. Unfortunately, the paper also advocated a controversial technology (flooding with aqueous colloidal dispersion gels) as being superior to polymer flooding. This claim is misleading and generally incorrect. “Colloidal dispersion gels” (“CDG” or relatively low concentrations of HPAM crosslinked with aluminum citrate) should not be applied without carefully examining the purported science and engineering behind this process.
Chang and the CDG vendor that he represents speculated that low-concentration aluminum-citrate-HPAM micro gels propagate through porous rock like super polymer solutions.1-4 Specifically, they suggested that these CDG formulations penetrate deep into porous matrix reservoir rock and subsequently provide higher resistance factors (effective viscosities in porous media) and residual resistance factors (permeability reduction factors) than comparable HPAM polymer solutions without crosslinker.
Because Chang’s paper was published in the “SPE Distinguished Author Series,” the Society of Petroleum Engineers may be perceived as endorsing colloidal dispersion gels as an accepted, state-of-the-art, better-than-polymer-flooding technology. The purpose of this report is to question that status.
Controversy Background
To understand this controversy, the reader must first recognize the distinction between a conformance treatment (i.e., permeability-reduction or blocking agent) and a polymer flood (mobility-control agent).5 Conventional gels used in "conformance control" are intended to block or reduce the flow capacity of high-permeability channels without damaging less-permeable hydrocarbon-productive zones (Fig. 1). In this situation, the objective is to minimize penetration of gelants or permeability-reducing agents into the less-permeable, oil-productive zones. Any gel or blocking agent that enters the less-permeable zones can hinder (or even shut off) subsequent injected fluids (e.g., water) from entering and displacing oil from those zones. In contrast, polymer floods and similar mobility-control methods are intended to directly displace oil from less-permeable zones (as well as improve mobility ratio and sweep in any given zone.) Consequently, a polymer solution should penetrate as much as possible into the less-permeable zones so that oil can be displaced from these poorly swept zones. For any material that enters the hydrocarbon zones, the engineer must ask, Will this damage the flow capacity of my hydrocarbon zone more than that of the water zone?
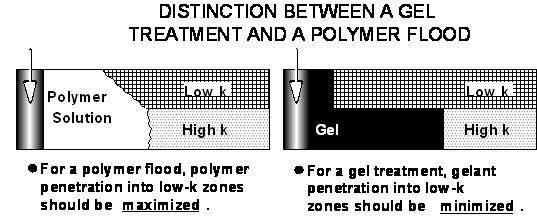
Polymers and Gelants Can Flow Through Rock
Consider how crosslinked polymer gels perform in porous media during conformance-improvement treatments.6-11 Early in the gelation process, most gelants (e.g., polymer-crosslinker solutions prior to significant polymer crosslinking) behave like clean fluids that do not contain suspended particulate matter.6-11 However, after the first gel aggregates form and grow to the size of pore throats, filtration of the micro-gel aggregates (within the porous rock) can radically increase the resistance to flow.6-8 Gelants can penetrate a significant distance into porous rock before gelation, but after gelation, gel propagation is extremely slow or non existent.6-11 The gelation onset time of aluminum-citrate-HPAM CDG formulations are relatively short (a few hours at 40-50°C). If gelation is stopped sufficiently early or if gels are sufficiently sheared so that gel particles remain significantly smaller than pore throats, the gel suspensions can propagate through porous rock; however, the level of mobility reduction (residual resistance factor) is generally small (less than 2).12 Independent studies at several locations (the University of Kansas,13-15 the University of Texas,16 New Mexico Tech,17,18 Stavanger College,19 and BP20) confirm that aluminum-citrate-HPAM gelants and gels behave like other gelants and gels.
Chang's Claim Violates Darcy's Law.
In Ref. 1, Chang claimed that "a large amount of CDG would preferentially enter the high-permeability or thief zones and divert polymer or water into medium- and low-permeability zones." This unsupported assertion contradicts basic calculations using Darcy's law, as demonstrated by Refs. 5, 21, and 22. To make these calculations readily accessible, the reader can download and use the second spreadsheet under "Unfractured Wells" at: Spreadsheets of important calculations. By experimenting with this spreadsheet, the reader should appreciate that the polymer or gelant DOES NOT "preferentially enter the high-permeability or thief zones." Instead, it penetrates into each zone in accordance with Darcy's Law. In fact, for a given volume of fluid injection, viscous solutions penetrate proportionately farther into low-permeability zones than water. That is a basic principle of polymer flooding and fluid displacement that has been known for many years. Table 1 gives an example to illustrate this point when no crossflow occurs between layers. If crossflow can occur, the distances of gelant penetration into the less-permeable zones (relative to that in the most-permeable zone) will be significantly greater. This point can be appreciated by viewing the videos here.
| Gelant radius, ft | ||||
| Layer | k,md | 1-cp gelant | 10-cp gelant | 40-cp gelant |
| 1 | 1,000 | 173.9 | 170.9 | 170.0 |
| 2 | 500 | 123.0 | 123.6 | 123.7 |
| 3 | 250 | 87.0 | 89.5 | 90.2 |
| 4 | 125 | 61.5 | 64.5 | 65.9 |
Polymers and Gels Plug Low-k Rock More than High-k Rock
Will the flow profile be improved if water is injected after the polymer or gel has been placed? Chang claims that after placement, the CDG gel will “divert water into medium- and low-permeability zones.” For radial flow, this claim is untrue. Suspensions of gel particles and adsorbed polymers [and adsorbed gel aggregates] provide resistance factors and residual resistance factors that increase with decreasing absolute permeability of the treated matrix reservoir rock.9,23-27 (Fig. 2 illustrates this fact for a common HPAM solution.) Not surprisingly, polymers and suspensions of small gel particles are more effective at restricting flow through small pore throats than through large pore throats. That is, the polymers or gel particles reduce the permeability of low-permeability reservoir matrix rock more than they reduce the permeability of the problematic high-permeability reservoir rock—the opposite of what is desired. Straightforward calculations using the Darcy equation reveal that this behavior is detrimental for sweep improvement—both during polymer flooding and gel treatments.5,21,22
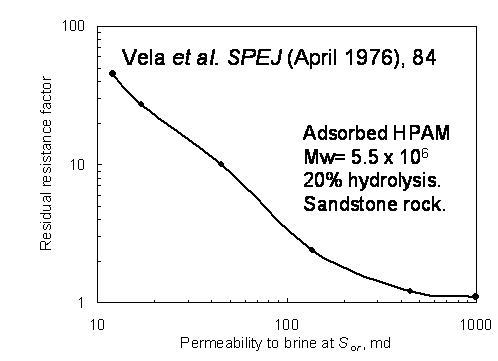
To appreciate this fact, the reader can again use the second spreadsheet under "Unfractured Wells" at Spreadsheets of important calculations. From Table 1, we used placement numbers (i.e., gelant radii) associated with the 10-cp polymer or gelant.
Three cases were considered. In each case, water was injected after polymer or gelant placement (again no crossflow), and for each of the four zones, we calculated the water injectivity index after polymer/gel placement relative to the value before placement (I/Io). Case 1, the most optimistic case, assumed that the residual resistance factors (Frr) were the same in all layers. For this case, the polymer treatment did reduce the flow capacity of Layer 1 slightly more than that of Layer 4 (0.86 versus 0.88). However, the improvement was so slight that it would not be considered of practical significance in most applications. Further, existing knowledge (e.g., Fig. 2) lends no support that residual resistance factors would be the same in all layers.
| Case 1 | Case 2 | Case 3 | ||||||
| Layer | k,md | Gelant radius,ft | Frr | I/Io | Frr | I/Io | Frr | I/Io |
| 1 | 1,000 | 170.9 | 1.5 | 0.86 | 1.1 | 0.97 | 1.5 | 0.86 |
| 2 | 500 | 123.6 | 1.5 | 0.86 | 1.2 | 0.94 | 2 | 0.68 |
| 3 | 250 | 89.5 | 1.5 | 0.87 | 1.8 | 0.82 | 2.5 | 0.63 |
| 4 | 125 | 64.5 | 1.5 | 0.88 | 2.3 | 0.75 | 3 | 0.59 |
For Case 2, data was taken from the literature (Ref. 24), representing measured residual resistance factors for a polyacrylamide (HPAM) solution as a function of permeability (Fig. 2). Here, the residual resistance factor increased from 1.1 to 2.3 as layer permeability decreased from 1,000 to 125 md. In this case, the flow profile was noticeably harmed by the HPAM solution—because I/Io decreased from 0.97 to 0.75 as permeability decreased from 1,000 to 125 md.
For Case 3, the residual resistance factor increased moderately with decreasing permeability (from 1.5 to 3 as layer permeability decreased from 1,000 to 125 md). In this case, which might be representative of the behavior of a CDG gel, the flow profile was significantly harmed by the polymer/gel treatment—because I/Io decreased from 0.86 to 0.59 as permeability decreased from 1,000 to 125 md. Thus, if the CDG claim of Ref. 1 was true (that CDG gels provide higher resistance factors and residual resistance factors than polymer alone), CDG gels could actually harm sweep efficiency.
In summary, if credible choices are made for the residual resistance factors (factors by which polymer or gel reduces permeability in the polymer- or gel-contacted rock), no significant improvement in sweep will result, beyond the benefit achieved with a normal polymer flood. During water injection after the polymer or CDG flood, sweep efficiency will not generally be better than before the polymer flood. If CDG gels truly provide greater residual resistance factors than uncrosslinked polymer, then CDG residual resistance factors should increase with decreasing permeability in a way that is more extreme than for polymer alone. Consequently, CDG gels can actually HARM sweep efficiency, when compared to a normal polymer flood. The best that can be reasonably hoped for is that the aluminum citrate does nothing—i.e., has no effect on the polymer. In that case, why waste money on the aluminum citrate?
Laboratory Results
Many Previous Lab Tests Show No Effect of Aluminum on Polymer
Independent tests from several university and industry laboratories confirm that aluminum-citrate-HPAM “colloidal dispersion” gelants and gels show the same basic traits as other gels used for conformance improvement.13-20 That is, they are NOT super-polymer flooding agents. In particular, researchers from the University of Kansas13,14 , the University of Texas16, and New Mexico Tech17,18 found that resistance factors (effective viscosities) provided by aluminum-citrate-HPAM colloidal dispersion gels within cores (i.e., beyond the inlet core section) were the same as those provided by polymer solution alone (i.e., containing no crosslinker). This observation suggests that either the gel particles were too small to interact significantly with pore throats or the crosslinking reaction did not take place to a significant extent. These possibilities are quite consistent with the behavior for other gels. If insufficient polymer or crosslinker is present, gel formation will not take place (even small gel particles may not form). With such low aluminum concentrations (as low as 15 mg/L), it is not surprising that a small (and expected) loss of crosslinker (e.g., by ion exchange or precipitation) could preclude formation of adequate gel particles.
Other Lab Tests Show Permeability Reductions, But Gelation Times Are Short
In other cases,15,17 CDG gelants were found to penetrate into sand packs or sandstone and ultimately provide significant residual resistance factors. However, relatively short gelation times (less than one day) preclude deep penetration into reservoirs (i.e., over the course of weeks or months).15,17
Parallel Corefloods Are a Poor Indicator of Diversion
Representing a vendor of the colloidal dispersion gels, Smith et al.3 argued in contradiction to the results published by the above independent laboratories. The work of Smith et al. focused on parallel linear corefloods,3 which can be easily manipulated and misinterpreted as suggesting successful fluid diversion.23 These laboratory test results cannot be directly translated to profile modification in radial flow (i.e., unfractured wells).21,23 Also, with short laboratory cores (as used in the above laboratory study), diffusion and dispersion can readily compromise small gelant banks placed in the less permeable of the parallel cores—giving the false impression that gelant does not significantly enter or damage less-permeable oil zones.22 In real field applications, the distance of gelant penetration is several feet or more, even in the least permeable oil-productive zones.5,21,22 For these distances, diffusion and dispersion will not destroy gelant banks.22
The Vendor’s Lab Tests Show No Surprises
Smith et al.3 also argued that the aluminum-citrate-HPAM gelants can effectively propagate deep into porous rock and still provide greater residual resistance factors (permeability reduction factors) than polymer alone. However, in their lab study, all gelant was injected (mostly at high rates) in less than 2.5 hours after gelant preparation. No internal pressure taps, along the core material length, were used in their work, so the degree of injection face-plugging versus in-depth gel propagation could not be assessed. Smith et al. argued that in-depth propagation was demonstrated because some of the effluent from the cores eventually formed gels. However, rapidly injected gelant would be expected to propagate through short cores and still form gels. Their results did not indicate that gelant (polymer AND crosslinker) would propagate for days, weeks, or months through porous rock—as required, if CDG flooding were to replace polymer flooding. Furthermore, their results did not indicate that gel will form and reduce permeability deep in the reservoir (beyond that caused by the polymer alone). As mentioned above, data from independent laboratories indicate that aluminum-citrate-HPAM gels of the type discussed in Refs. 1-4 will not propagate deep into porous rock and still provide greater residual resistance factors than polymer alone.
Requirements for a Viable Colloidal Dispersion Gel
If one wished to develop and demonstrate positive behavior for a suspension of gel particles, a number of useful and informative experiments should be performed. First, experiments should be performed using cores with multiple sections (e.g., multiple internal pressure taps along the core’s length). The gelant formulation must (1) be injectable into the rock without causing progressive plugging of the inlet sand face, (2) show uniform resistance factors and residual resistance factors along the entire length of the core, (3) propagate these resistance factors through the porous rock at an acceptable rate (i.e., no excessive chemical retention), AND (4) provide greater resistance factors/residual resistance factors than polymer solution alone. During this series of flooding-experiment studies, one must also be concerned about the magnitude of residual resistance factors as a function of rock permeability.23-27 This latter comparison should be made using cores that were completely filled with polymer or gel (i.e., NOT using misleading parallel corefloods). If residual resistance factors in low-permeability rock are significantly greater than in high-permeability rock, polymer or gel treatments will impair sweep efficiency (see Fig. 2 and Table 2).5,21
Examination of Field Data
The Field and Lab Data Are Right. Only Interpretations Are Wrong
To CDG customers, the most influential claim made by those who advocate use of colloidal dispersion gels is that “successful” field applications demonstrate their utility.1-4 The CDG vendor suggested that a discrepancy exists between laboratory and field results—and that field results justify their untenable explanations. However, Darcy’s Law, principles of polymer flooding, and the behavior of gelants and gels in porous media are very well established. Proposed explanations for field results should not contradict these principles without good reason.
If Darcy’s Law and independent and well-documented laboratory results are accepted, two lines of reasoning remain to be investigated for explaining CDG field results. One line of reasoning is that CDG gels provided a benefit through some unrecognized mechanism. The challenge here is to identify any possible new mechanism. The second line of reasoning is that the aluminum citrate provided no significant benefit, even though some benefit may have come from using the polymer. In other words, is it possible that the aluminum citrate gave no incremental benefit over using the polymer alone? We are investigating both possibilities.
Field Results Can Be Explained Using More Plausible Concepts
In our examination of CDG projects in the United States and elsewhere, we have yet to see a convincing case that supports the untenable claims made by Chang and the CDG vendor (i.e., that CDG gels act as super polymer solutions and that CDG gels preferentially enter high-permeability strata and later divert water into low-permeability zones). Instead, credible explanations can be envisioned that are consistent both with field and laboratory findings.
In general, the CDG field results fit into one of several categories. In the first field-results category, the treated reservoirs (with matrix rock permeabilities less than 10,000 md) contained no fractures or fracture-like flow features. For these cases, the aluminum was probably either removed (by adsorption on rock or precipitation) or for some other reason did not crosslink the polymer. So the polymer alone could propagate (in an uncrosslinked form) through the formation and provide some benefit as a polymer flood. This scenario is very consistent with the laboratory findings from the University of Texas16 and the University of Kansas.14 Since the aluminum provided no benefit for this scenario, the money spent on aluminum citrate could have been more effectively spent on more polymer.
In the second field-results category, the treated wells contained fractures or fracture-like flow features (e.g., vugs, karst, very-permeable conglomerate), even though the operator or gel vendor may not have been aware of these high-permeability flow features before the treatments. For these cases, the gels may have provided some benefit by partially plugging the fractures, fracture-like features, or high-permeability anomalies. However, for moderate to wide fractures, other types of gels [e.g., Cr(III)-acetate-HPAM)] probably would have been more effective. On a positive note for narrow (e.g., micro) fractures, colloidal dispersion gels conceivably may be more effective than other gels because of more effective penetration into tight fractures.28,29 This possibility requires further investigation.
In the third field-results category, the treated wells experienced general plugging of all open zones and flow paths. From an areal view, these wells were key to water channeling. By reducing the flow capacity of the treated wells, areal pressure gradients were altered so water injected into other (non-treated) wells pushed incremental oil toward offset production wells. This benefit could be realized by any means that reduced the flow capacity of the treated wells—including just choking back the injection rate of the well. Injection-well flow capacity can usually be reduced more cost-effectively and more easily using methods other than the CDG technology.
In the fourth field-results category, the reported benefits and/or increases in oil recovery had nothing to do with the colloidal dispersion gels. In some cases, the reported benefits for particular wells and fields occurred because of other changes or improvements that were implemented. In other cases, no real benefit occurred. The reported benefit was an artificial result of an overly pessimistic projection of the pre-treatment decline curve and/or an overly optimistic assignment of incremental oil to the gel treatment.
Daqing Field Results Are Consistent with No Benefit from Aluminum Citrate
Careful objective analysis of the Daqing CDG field data suggest no credible, unambiguous improvement of the “CDG” flood over the normal polymer flood (especially see Tables 13 and 15 and Figs. 3, 4, 10, and 11 in Ref. 2). Injectivity behavior was not significantly different during CDG injection versus polymer injection. Also, water/oil ratios and production trends cannot be credibly or unambiguously distinguished for the two processes. These results are consistent with the first category of field results mentioned above: the aluminum was either removed (by adsorption or precipitation) or was present in concentrations too low to allow polymer crosslinking within the reservoir.
In Table 13 of Ref. 2, Chang listed resistance factors of 1.316 (increasing from 1.120 to 1.512) during injection of a 0.179 PV CDG Slug 1, 1.50 during injection of a subsequent 0.155 PV polymer bank (with the same polymer concentration), and 1.537 during injection of a final 0.196 PV CDG Slug 2. Chang2 claimed “indeed, the CDG solution formed strong resistance deep in the reservoir.” Several serious problems are evident with this statement. First, it is not credible that resistance factors could be measured to three decimal places using field data—or even using lab data. Measurements accurate to 1 decimal place are normally the best that could be hoped for. Second, it is not credible that a resistance factor of 1.50 is significantly different from 1.512 or 1.537. (In truth, considering normal field data, it is difficult to believe that a value of 1.5 is statistically different from 1.3). Third, why was the resistance factor only around 1.5 when the solutions injected had viscosities around 20 cp? Why wouldn’t the resistance factor be at least 20 cp? The fact that the resistance factors were so low suggests a deficiency in the method of measurement.
The similarity of behavior during polymer and CDG (polymer with aluminum citrate) injection was evident in many of the tables and figures in Ref. 2 (see Tables 13 and 15 and Figs. 3, 4, 10, and 11). These observations suggest that the aluminum citrate provided no benefit over normal polymer flooding.
More Convincing Field Data Needed
Undoubtedly, Chang and the CDG vendor that he represents feel that their CDG process works better than polymer floods and that we have missed some important mechanism of action during this discussion. We encourage them to pursue and demonstrate their point. However, future demonstrations should include two important aspects. First and foremost, explanations for how the process works should be consistent with well-established physical principles (e.g., Darcy’s law). Second, if they feel that field results are demonstrating some new physical principle, the field results should be less ambiguous than those presented in the past.
Searching for a New Mechanism of Action for CDG Gels
Does Shearing Of Colloidal Dispersion Gels Allow For Effective, Deep Penetration?
Vendor Speculation
The CDG vendor speculated that when CDG gelants are sheared at high velocities in rock near a wellbore, gelation is delayed substantially (i.e., by weeks or months)—thus, allowing gelant to penetrate far into a formation before developing high resistance when flowing at low velocities.3 This was pure speculation—no credible data was provided to support the suggestion.
First Test: Polymer Solution Injection
To test the vendor’s hypothesis, we performed two sets of two-part experiments. CDG formulations were prepared that contained 300-ppm HPAM (Tiorco HiVis 350™), 15-ppm aluminum (as citrate, Tiorco 677™), and 0.5% KCl. All experiments were performed at 41°C. In the first set of experiments, 300-ppm HPAM (without aluminum) were forced through a 493-md, 0.43-ft-long (5.2-inches) Berea core using a superficial velocity of 143 ft/d. This core had one internal pressure tap that was located 0.75 inches from the inlet sand face.
During the course of injecting 118 PV (3,800 cm3) of polymer solution,the resistance factors (apparent viscosities) were fairly stable andabout the same (~42) in both core sections (left side of Fig. 3). Thepolymer effluent from this short core was collected and then injectedinto a 234-md, 4-ft-long Berea core using a much lower rate of 2.7 ft/d.This core had four equally spaced internal pressure taps, dividing thecore into five sections of equal length. When the polymer solution effluentfrom the short core was injected into the long core, resistance factorswere fairly similar in all five core sections—averaging about 7(left side of Fig. 4). Incidentally, resistance factors for HPAM solutionsare well known to increase with increased velocity in porous media30—consistentwith our value of 42 in the short core at 143 ft/d versus 7 in the longcore at 2.7 ft/d. This effect has been attributed to the viscoelastic character of the HPAM polymer.30
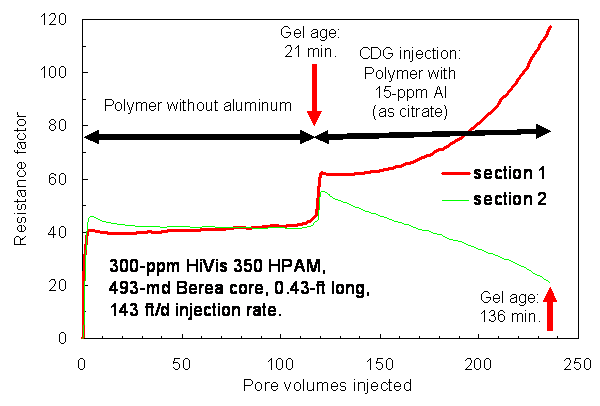
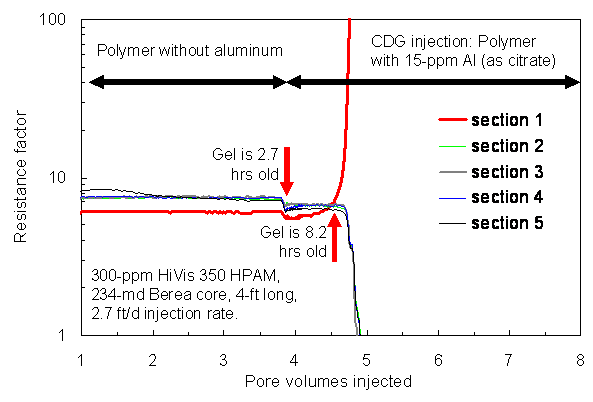
First Test: CDG Injection
After injection of polymer solution, CDG formulation with the same polymer concentration (300-ppm HPAM) and with 15-ppm aluminum were injected into the 493-md, 0.43-ft-long Berea core, again at a rate of 143 ft/d. For the first experiment (right side of Fig. 3), the CDG gel was 21 minutes old at the start of gelant injection and 136 minutes old at the end of gelant injection. During injection of 118 PV, the resistance factor increased to 117 in the first core section and decreased to 21 in the second core section. We concluded that some of the CDG gel was stripped from the solution during flow through the first core section. CDG gelant that was effluent from the first core was 163 minutes (2.7 hrs) old at the start of gelant injection into the 4-ft long core. For the next 5.5 hrs (0.8 PV) of CDG injection (middle of Fig. 4), resistance factors remained stable in the five core sections. However, thereafter the resistance factor in the first core section quickly rose to a very high value, indicating severe face-plugging by the CDG gel. Simultaneously, resistance factors in the other four sections dropped to low values, indicating that the solution flowing through the downstream sections had been stripped of polymer. Thus, the concept advocated by the vendor does not appear to be valid.
Second Test: Polymer Solution Injection
To confirm the above results, these experiments were repeated. In the second set of experiments, 300-ppm HPAM (without aluminum) were forced through a 506-md, 0.43-ft-long Berea core using a superficial velocity of 138 ft/d. This core had one internal pressure tap that was located 0.83 inches from the inlet sand face. During the course of injecting 123 PV (3,870 cm3) of polymer solution, the resistance factors (apparent viscosities) were not as stable as those from the first set of experiments—steadily rising to 45 in the first section but decreasing to 28 in the second core section (left side of Fig. 5). The polymer effluent from this short core was collected and then injected into a 196-md, 4-ft-long Berea core using a much lower rate of 1.1 ft/d. As in the first set of experiments, this core had four equally spaced internal pressure taps, dividing the core into five sections of equal length. When the polymer solution effluent from the short core was injected into the long core, resistance factors (left side of Fig. 6) were fairly similar in all five core sections—averaging about 6, close to the value of 7 observed during the first set of experiments (left side of Fig. 4).
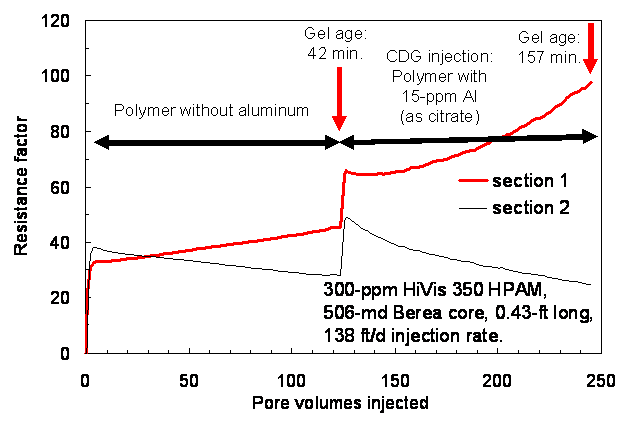
Second Test: CDG Injection
After injection of polymer solution, CDG formulation with the same polymer concentration (300-ppm HPAM) and with 15-ppm aluminum were injected into the 506-md, 0.43-ft-long Berea core, again at a rate of 138 ft/d. For the first experiment (right side of Fig. 5), the CDG gel was 42 minutes old at the start of gelant injection and 157 minutes old at the end of gelant injection. During injection of 115 PV, the resistance factor increased to 98 in the first core section and decreased to 24 in the second core section. This behavior was similar to that seen during the analogous portion of the first set of experiments.
CDG gelant that was effluent from the first core was 192 minutes (3.2 hrs) old at the start of gelant injection into the 4-ft long core. For the next 5.7 hrs (0.4 PV) of CDG injection (right side of Fig. 6), resistance factors remained stable in the final four core sections. However, the resistance factor in the first core section quickly rose to a very high value, indicating severe face-plugging by the CDG gel. After 0.4 PV of CDG injection, resistance factors in the other four sections dropped to low values, indicating that solution flowing through the downstream sections had been stripped of polymer. These results were reasonably consistent with those during the first set of experiments. Thus, the concept advocated by the vendor does not appear to be valid.
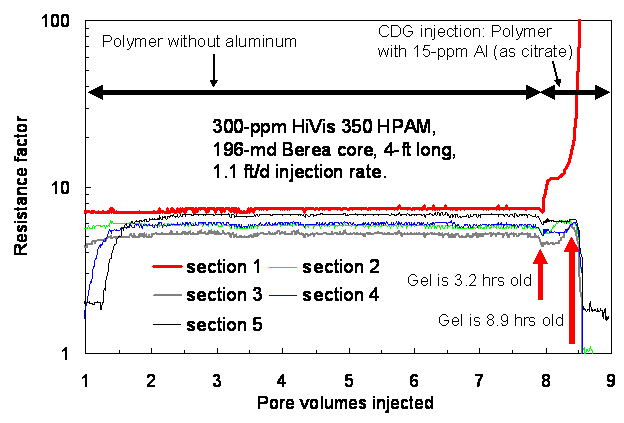
Conclusion
Shearing CDG gelants through porous rock may delay gelation and development of high resistance factors by a few hours, but certainly NOT for days, weeks, or months, as speculated (without support) by the CDG vendor. A relatively short time after gelant preparation (8.2 to 8.9 hrs), the sheared CDG gel caused severe plugging and did not propagate through 196 to 234-md rock.
Can CDG Gels Flow in Porous Media with Permeabilities Less than 8 Darcys?
Presumably, formed colloidal dispersion gels (or any other gel) could flow through a porous medium if the permeability was sufficiently high or if the pressure gradient was sufficiently large. For an extreme example, if the porous medium consisted of packed bowling balls, we suspect that many gels could readily be extruded through. Reservoir strata have been reported that have matrix permeabilities between 1 and 10 darcys—where fractures, vugs, and fracture-like features are not present.
Experimental
We wondered whether formed CDG gels could enter and flow through a consolidated porous medium with a permeability up to 10 darcys. A 7.9-darcy porous polyethylene core was cast that was 2.6 ft (78.4 cm) long and 1.55 inches (3.94 cm) in diameter. Porosity was 36.5%. Four internal pressure taps were equally spaced along the core, creating five core sections of equal length. The core was saturated with brine (0.5% KCl). All experiments were performed at 41°C. A colloidal dispersion gel was prepared that contained 300-ppm Tiorco HiVis350 HPAM, 15-ppm aluminum (as citrate, Tiorco 677), and 0.5% KCl. This formulation was aged for one day at 41°C. Then it was injected into the core using a rate of 26 ft/d (400 cm3/hr).
Results
Fig. 7 shows that resistance factors in the first core section rapidly rose and exceeded 1,000 during the first 0.08 PV of CDG injection. At this point (4 minutes after the start of injection), the throughput value for the inlet face was 2.2 cm3/cm2, and the pressure drop across the first core section was 179 psi. Thus, the gel caused severe face plugging. In contrast, resistance factors for the other four sections of the core remained low–indicating no propagation of CDG gel beyond the first core section.

Conclusion
1-day-old colloidal dispersion gels do not flow through porous media with permeabilities less than 8 darcys.
Can CDG Gels Be More Effective than Normal Gels in Tight Fractures?
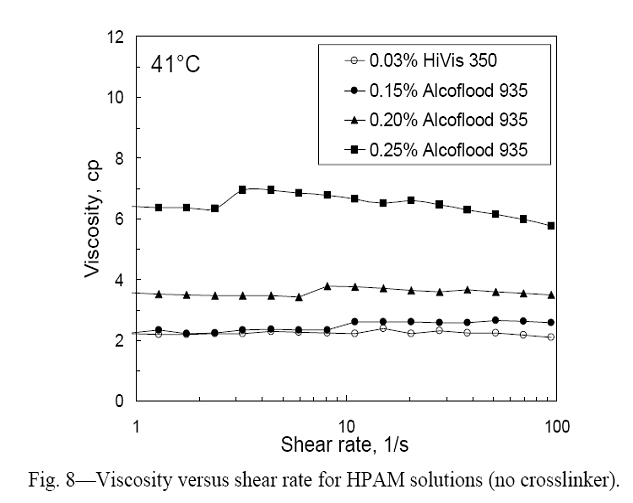
Can low concentration gels penetrate into and plug narrow fractures more effectively than more concentrated gels? Our previous work revealed that the pressure gradient required to extrude a gel through a fracture varied inversely with the square of fracture width.32 For a one-day-old Cr(III)-acetate-HPAM gel with 0.5% Alcoflood 935, 0.0417% Cr(III) acetate, 1% NaCl, and 0.1% CaCl2, the pressure gradient needed for extrusion through a 0.1-mm-wide fracture was over 1,000 psi/ft. During another set of experiments, we noted that a "partially formed" (i.e., gelant aged slightly longer than the gelation time) Cr(III)-acetate-HPAM gel with 0.5% HPAM did not penetrate into a 0.05-mm-wide fracture with a pressure gradient of 65 psi/ft.33 So for pressure gradients that are representative of field applications, Cr(III)-acetate-HPAM gels with concentrations of 0.5% HPAM or more will not penetrate significant distances into narrow fractures. We wondered whether low concentrations of gel might show value in penetrating into and plugging tight fractures more effectively than conventional gels with higher concentrations. (Baojun Bai et al. performed preliminary work investigating this idea.28,29) We performed experiments using gels with four compositions: (1) 0.03% Tiorco HiVis 350 HPAM and 0.0023% Al(III) citrate (0.0015% Al), (2) 0.15% Alcoflood 935 HPAM and 0.0125% Cr(III) acetate (0.0028% Cr), (3) 0.2% Alcoflood 935 HPAM and 0.0167% Cr(III) acetate (0.0037% Cr), and (4) 0.25% Alcoflood 935 HPAM and 0.0209% Cr(III) acetate (0.0047% Cr). The first formulation contained 0.5% KCl while the other three formulations contained 1% NaCl and 0.1% CaCl2. All experiments were performed at 41°C. Fig. 8 shows viscosity versus shear rate for HPAM solutions with no crosslinker. Within the experimental error, the viscosities were fairly Newtonian for shear rates from 1 to 100 s-1, exhibiting average viscosities of 2.2 cp for 0.03% HiVis 350 HPAM, 2.5 cp for 0.15% Alcoflood 935 HPAM, 3.5 cp for 0.2% Alcoflood 935 HPAM, and 6.5 cp for 0.25% Alcoflood 935 HPAM. Incidentally, our interest in low concentration Cr(III)-acetate-HPAM gels arose because of work performed in Argentina,34 where Tiorco appears to be applying the Cr(III)-acetate-HPAM gels with similar objectives as those for their aluminum-citrate-HPAM CDG gels.
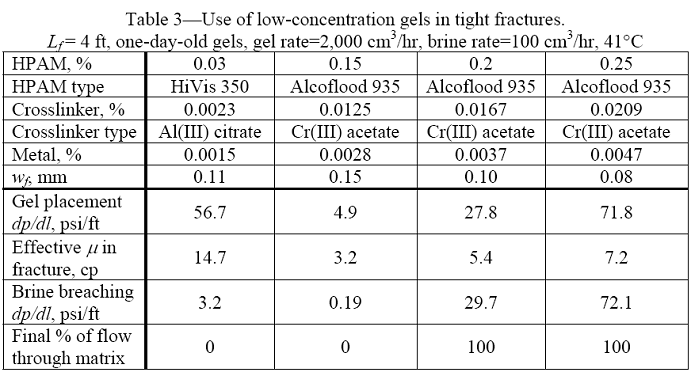
With each gel composition, we performed experiments where 3,700 cm3 of one-day-old gel were extruded through fractures at 2,000 cm3/hr. All fractures were 3.8 cm high in Berea sandstone cores that were 4 ft (122 cm) long and 11.4 cm2 in cross-section. Four internal pressure taps (drilled into the fracture) divided the core into five sections of equal length (0.8 ft). For each core, effluent could be produced from both the matrix and the fracture. The experiments used closed fractures, with calculated fracture widths ranging from 0.08 to 0.15 mm. All fractures had smooth-sawed faces. After gel placement, brine was injected at a rate of 100 cm3/hr. Table 3 summarizes the results.
Behavior during Gel Injection
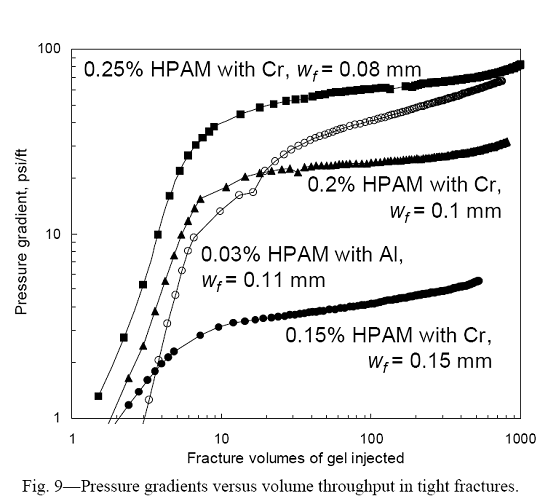
As mentioned, the gel formulations were aged for one day before injection into the fractured cores at a rate of 2,000 cm3/hr. Fig. 9 plots pressure gradient versus gel throughput (expressed in fracture volumes) for the four gels. Gel was detected at the fracture outlets after injecting from 3 to 10 fracture volumes. (One fracture volume = 4.6 cm3 if wf = 0.1 mm.) Between 10 and 500 fracture volumes of gel throughput, pressure gradients increased by 60 to 85% for the Cr(III)-acetate-HPAM gels. In contrast, for the Al(III)-citrate-HPAM CDG gel, the pressure gradient increased by 460%. The first data row below the solid line in Table 3 lists the average pressure gradients during gel extrusion. As expected, the average pressure gradient increased significantly with increased HPAM concentration for the Cr(III)-acetate-HPAM gels. Interestingly, the average pressure gradient for the Al(III)-citrate-HPAM CDG gel was quite high (56.7 psi/ft), considering that the gel contained only 0.03% HPAM. This result may have occurred because the molecular weight of HiVis 350 is significantly greater than that for Alcoflood 935.
The second data row below the solid line in Table 3 lists the effective viscosity exhibited by the gel in the fractures. For the Cr(III)-acetate-HPAM gels, the effective viscosities were relatively low–on the order of the viscosities of the uncrosslinked polymer solutions (Fig. 8). These low effective viscosities should aid deep placement in narrow fractures. For the Al(III)-citrate-HPAM CDG gel, the effective viscosity in the fracture (14.7 cp) was significantly greater than the polymer viscosity (2.2 cp).
Behavior during Brine Injection after Gel Placement
The second to last row in Table 3 lists the maximum pressure gradient observed during the first brine injection (at 100 cm3/hr) after gel placement. This pressure gradient indicates the point where brine first breached the gel.35 For the two cases with 0.2% or more of HPAM, the brine breaching pressures were significant and on the order of the pressure gradients during gel placement. For the two cases with less than 0.2% HPAM, the brine breaching pressures were significantly less than the pressure gradients during gel placement.
The last row indicates that fraction of the injected brine that flowed through the matrix. The last row is particularly indicative of the diversion properties of the gel. Note that in two cases involving gels with 0.2% HPAM or 0.25% HPAM, 100% of the brine flow occurred through the matrix (i.e., 0% of the flow occurred through the fracture) after the gel treatment. For the other cases, little or no brine flowed through the matrix after the gel treatment. Thus, the two gels with higher HPAM concentrations were much more effective in plugging the tight fractures than those with the lower HPAM concentrations.
Conclusions
In fractures with widths around 0.1 mm, one-day-old Cr(III)-acetate-HPAM gels containing 0.15%, 0.2%, or 0.25% HPAM propagated effectively, exhibiting effective viscosities that were similar to the viscosity of polymer solutions without crosslinker. In contrast, our previous work revealed that Cr(III)-acetate-HPAM gels with 0.5% HPAM would not enter these narrow fractures unless extremely high pressure gradients were applied. The gels containing 0.2% or 0.25% HPAM effectively healed these narrow fractures, forcing all post-gel-treatment brine to flow through the Berea sandstone matrix rather than the narrow fractures. Gels with lower HPAM concentrations were ineffective in preventing channeling through the fractures. An Al(III)-citrate-HPAM CDG gel exhibited relatively high effective viscosity when extruding through a tight fracture and was ineffective in preventing channeling through the fracture. The Al(III)-citrate-HPAM CDG gel appeared less attractive than Cr(III)-acetate-HPAM gels for treating tight fractures.
Summary
In summary, the suggestion 1,2 that the CDG process was superior to normal polymer flooding was misleading and generally incorrect. Colloidal dispersion gels, in their present state of technological development, should not be advocated as an improvement to, or substitute for, polymer flooding.
References
- Chang, H.L., et al.: "Advances in Polymer Flooding and Alkaline/Surfactant/Polymer Processes as Developed and Applied in the People's Republic of China," JPT (February 2006) 84-89..
- Chang, H.L., et al.: "Successful Field Pilot of In-Depth Colloidal Dispersion Gel (CDG) Technology in Daqing Oil Field," SPE paper 89460 presented at the 2004 SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, April 17-20.
- Smith, J.E., Liu, H., and Guo, Z.D.: "Laboratory Studies of In-Depth Colloidal Dispersion Gel Technology for Daqing Oil Field," SPE paper 62610 presented at the 2000 SPE/AAPG Western Regional Meeting, Long Beach, CA, June 19-23.
- Mack, J.C. and Smith, J.E.: "In-Depth Colloidal Dispersion Gels Improve Oil Recovery Efficiency," paper SPE 27780 presented at the 1994 SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, April 17-20.
- http://www.prrc.nmt.edu/groups/res-sweep/
- Hejri, S. et al.: "Permeability Reduction by a Xanthan/Cr(III) System in Porous Media," SPERE (Nov. 1993) 299-304.
- Todd, B.J., Green, D.W., and Willhite, G.P.: "A Mathematical Model of In-Situ Gelation of Polyacrylamide by a Redox Process," SPERE (February 1993) 51-58..
- Seright, R.S.: "Gel Placement in Fractured Systems," SPEPF (Nov. 1995), 241-248.
- Seright, R.S.: "Effect of Rock Permeability on Gel Performance in Fluid-Diversion Applications," In Situ (1993) 17 (4), 363-386.
- Seright, R.S. and Martin, F.D.: "Impact of Gelation pH, Rock Permeability, and Lithology on the Performance of a Monomer-Based Gel," SPERE (February 1993) 43-50.
- Seright, R.S. and Martin, F.D.: "Effect of Cr3+ on the Rheology of Xanthan Formulations in Porous Media: Before and After Gelation," In Situ (1992) 16 (1), 1-16.
- Rousseau, D. et al.: "Rheology and Transport in Porous Media of New Water Shutoff/Conformance Control Microgels," paper SPE 93254 presented at the 2005 SPE International Symposium on Oilfield Chemistry, Houston, TX, February 2-4.
- Rocha, C.A. et al.: "An Experimental Study of the Interactions of Aluminum Citrate Solutions and Silica Sand," paper SPE 18503 presented at the 1989 SPE International Symposium on Oilfield Chemistry, Houston, TX, February 8-10.
- Ranganathan, R. et al.: "Experimental Study of the Gelation Behavior of a Polyacrylamide/Aluminum Citrate Colloidal-Dispersion Gel System," SPEJ (Dec. 1998) 337-343.
- Al-Assi, A.A. et al.: "Formation and Propagation of Gel Aggregates Using Partially Hydrolyzed Polyacrylamide and Aluminum Citrate," paper SPE 100049 presented at the 2006 SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, April 22-26.
- Walsh, M.P. et al.: "Chemical Interactions of Aluminum-Citrate Solutions with Formation Minerals," paper SPE 11799 presented at the 1983 SPE International Symposium on Oilfield Chemistry, Denver, CO, June 1-3.
- Seright, R.S.: "Improved Techniques for Fluid Diversion in Oil Recovery Processes," second annual report (DOE/BC/14880-10), Contract No. DE-AC22-92BC14880, U.S. DOE (March 1995), 51-64.
- Wang, D., et al.: "Sweep Improvement Options for the Daqing Oil Field," paper SPE 99441 presented at the 2006 SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, April 22-26.
- Stavland, A. and Johsbraten, H.C.: "New Insight into Aluminum Citrate/Polyacrylamide Gels for Fluid Control," paper SPE/DOE 35381 presented at the 1996 SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, April 21-24.
- Fletcher, A.J.P. et al.: "Deep Diverting Gels for Very Cost-Effective Waterflood Control," J. Petr. Sci. Eng. 7 (1992) 33-43.
- Seright, R.S.: "Placement of Gels to Modify Injection Profiles," paper SPE/DOE 17332 presented at the 1988 SPE/DOE Enhanced Oil Recovery Symposium, Tulsa, OK, April 17-20.
- Seright, R.S.: "Impact of Dispersion on Gel Placement for Profile Control," SPERE (Aug. 1991) 343-352.
- Seright, R.S.: "Impact of Permeability and Lithology on Gel Performance," paper SPE 24190 presented at the 1992 SPE/DOE Symposium on Enhanced Oil Recovery, Tulsa, OK, April 22-24.
- Vela, S., Peaceman, D.W. and Sandvik, E.I.: "Evaluation of Polymer Flooding in a Layered Reservoir with Crossflow, Retention, and Degradation," SPEJ, 16(2), (April 1976) 82-96.
- Jennings, R.R., Rogers, J.H., and West, T.J.: "Factors Influencing Mobility Control by Polymer Solutions," JPT, 23(3), (Mar. 1971) 391-401.
- Hirasaki, G.J., and Pope, G.A.: "Analysis of Factors Influencing Mobility and Adsorption in the Flow of Polymer Solution Through Porous Media", SPEJ, 14(4), (Aug. 1974) 337-346.
- Zaitoun, A., and Kohler, N.: Two-Phase Flow Through Porous Media: Effect of an Adsorbed Polymer Layer," paper SPE 18085 presented at the 1988 SPE Annual Technical Conference and Exhibition, Houston, TX, Oct. 2-5.
- Bai, B. et al.: "Preformed Particle Gel for Conformance Control: Factors Affecting its Properties and Applications," paper SPE 89389 presented at the 2004 SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, April 17-21.
- Bai, B. et al.: "Preformed Particle Gel for Conformance Control: Transport Mechanism Through Porous Media," paper SPE 89468 presented at the 2004 SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, April 17-21.
- Seright, R.S.: "The Effects of Mechanical Degradation and Viscoelastic Behavior on Injectivity of Polyacrylamide Solutions," Society of Petroleum Engineers J. (June 1983) 475-85.
- Wang, D et al.: "Producing More than 75% of Daqing Oil Field's Production by IOR, What Experiences Have Been Learnt?", paper SPE 77871 presented at the 2002 SPE Asia Pacific Oil and Gas Conference and Exhibition, Melbourne, Australia, Oct. 8-10.
- Seright, R.S.: "An Alternative View of Filter Cake Formation in Fractures Inspired by Cr(III)-Acetate-HPAM Gel Extrusion," SPE Production and Facilities (Feb. 2003) 65-72.
- Seright, R.S.: "Conformance Improvement Using Gels," Annual Technical Progress Report (U.S. DOE Report DOE/BC/15316-6), U.S. DOE Contract DE-FC26-01BC15316 (Sept. 2004) 72.
- Norman, C., De Lucia, J., and Turner, B.: "Improving Volumetric Sweep Efficiency With Polymer Gels in the Cuyo Basin of Argentina," paper SPE 99379 presented at the 2006 SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, April 22-26.
- Seright, R.S.: "Washout of Cr(III)-Acetate-HPAM Gels from Fractures," paper SPE 80200 presented at the 2003 SPE International Symposium on Oilfield Chemistry, Houston, TX, Feb. 5-7.