Can diffusion be exploited to dilute the gelant bank in low-permeability zones enough to prevent gelation, while allowing an effective gel plug to form in high permeability zones?
Only if the gelant banks are extremely small—< 1 ft.
In concept, diffusion and dispersion could dilute gelants enough to prevent gelation in less-permeable, oil-productive zones while still allowing a gel plug to form in watered-out, high-permeability streaks. Whether or not a chemical bank can be diluted enough by diffusion to prevent gelation depends on at least four factors: 1) the size of the chemical bank, 2) the diffusion coefficient, 3) the gelation time, and 4) the extent of dilution required to prevent gelation.
Diffusion coefficients (D) are typically on the order of 10-5 cm2/s for low-molecular-weight chemicals in water.34 These chemicals include gelants such as acrylamide monomer, phenol, and formaldehyde. Diffusion coefficients are typically on the order of 10-8 cm2/s for high-molecular-weight polymeric gelants such as polyacrylamide or xanthan.37For low-molecular-weight species in a viscous polymer solution (e.g., Cr2O7-2 in water with 0.2% polyacrylamide), the diffusion coefficient should have some intermediate value-varying inversely with the viscosity of the solution.38
Gelation times range from a few minutes to several days for most formulations that have been considered for near-wellbore gel treatments. In general, the gelation time decreases with increasing concentrations of the gelling agents.39,40 Also, some minimum concentration of the proper reactants must be present in order for gelation to occur. In most field applications of gel treatments, the concentrations of reactants that are injected are well above the minimum levels required for gelation. Thus, significant dilution (often by a factor of two or more) is required in order to prevent gelation.
To be conservative, we assume that the gelation reaction is stopped by only a ten percent dilution of the reactants. Thus, the reader should bear in mind that the reductions in gel-bank size that are forecast due to dilution by diffusion will be overly optimistic. By overestimating the impact of diffusion in our analysis, we increase confidence in our major conclusion from this study. That is, in field applications in unfractured wells, diffusion will not usually cause enough dilution to prevent gelation in the less-permeable zones.
In field applications of gel treatments, wells are commonly shut-in for some time after injection of the gelant to allow the gel to form. During the time prior to gelation, diffusion acts to dilute the chemical banks. The size of the mixing zone, Lm, created by diffusion alone (no dispersion) during this time can be approximated using Eq. 5,
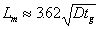
where tg is gelation time. The mixing zone given by Eq. 5 extends from the point where the gelant has been diluted to 90 percent of the original concentration to the point where the gelant has been diluted to 10 percent of the original concentration.36 Fig. 13 illustrates a typical concentration profile that results when diffusion or dispersion smears an interface that was originally sharp. Fig. 13 also illustrates the size of the mixing zone that is given by Eq. 5.
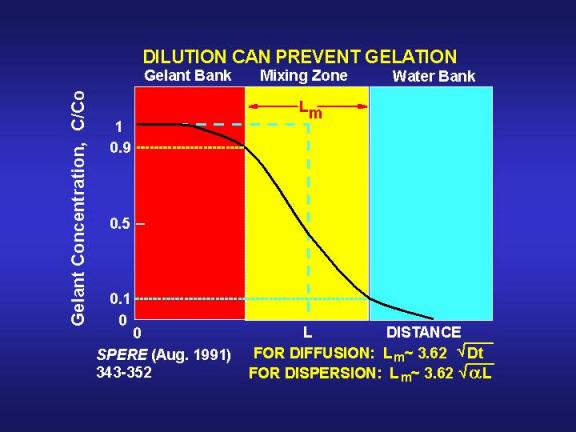
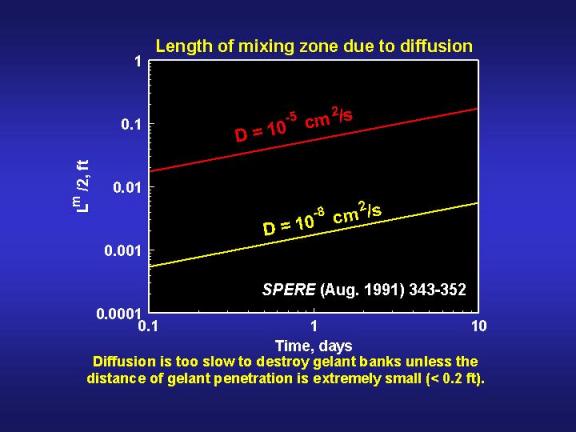
If the gelation reaction is stopped by a ten percent dilution of the reactants, then diffusion will reduce the gel bank size by the distance Lm/2. Fig. 14 provides values of Lm/2 as a function of time and diffusion coefficient. A key point illustrated by Fig. 14 is that diffusion will not create a large mixing zone in the period associated with typical gelation times. Even for relatively large diffusion coefficients (10-5cm2/s), Lm/2 is only about 0.2 ft (5 cm) after ten days. Considering the depths of penetration for gelants in typical field applications (see Fig. 9), diffusion is not likely to have a significant impact in unfractured wells. However, in fractured wells where the gelant leaks off a very short distance from the fracture faces, diffusion could have an important impact.
Diffusion can significantly affect results from parallel laboratory corefloods. Consider injection of a 1-cp gelant to displace water from two one-foot-long cores that are being flooded in parallel. Assume that one core is ten times more permeable than the other and both cores have the same porosity. When the gelant reaches the outlet of the most-permeable core, the gelant will have penetrated 0.1 ft into the less-permeable core. Over the course of one day, most of the gelant in the less-permeable core could be diluted if the diffusion coefficient is 10-5 cm2/s. Therefore, diffusion during gel placement in parallel laboratory corefloods can mislead one to conclude that zone isolation is not needed during gel placement in field projects.